Introduction to Formic Acid (HCOOH)
Formic acid (chemical formula HCOOH) is the simplest carboxylic acid, often found in nature and widely used in various industries. Its name comes from the Latin word Formica, meaning ant, because it was first isolated from ants. In nature, formic acid plays an essential role in ant venom, serving as a defensive and offensive chemical. Besides insects, small quantities of formic acid exist in some plants and natural processes. HCOOH is not just important; it has found applications in textiles, agriculture, leather production, and even pharmaceuticals.
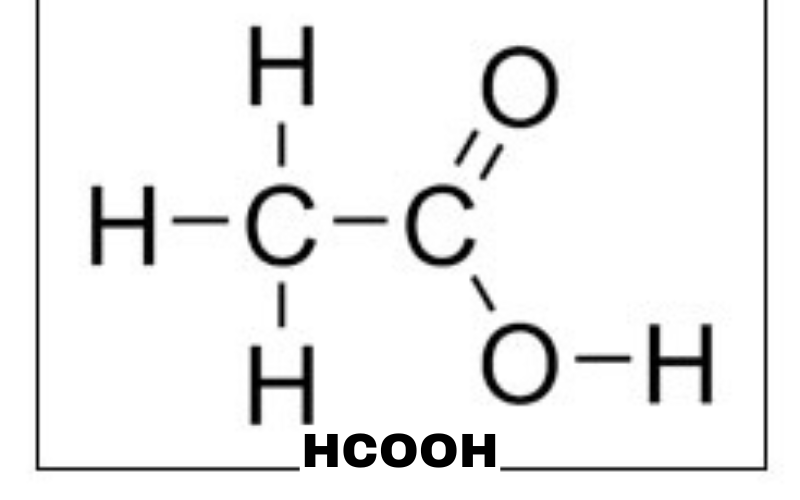
Formic Acid Chemical Formula and Molecular Structure
The molecular formula of formic acid is HCOOH, representing a simple one-carbon carboxylic acid. Structurally, formic acid contains a carbonyl group (C=O) attached to a hydroxyl group (-OH), making it both an aldehyde and a carboxylic acid. This dual functionality gives HCOOH some unique chemical properties. The molecular weight of formic acid is approximately 46.03 g/mol, and its structure allows for high solubility in water and polar solvents. Its simplicity makes it a fundamental compound for understanding organic acid behavior, especially when comparing formic acid with other carboxylic acids like acetic acid.
Physical and Chemical Properties of Formic Acid
Formic acid (HCOOH) is a clear, colorless liquid with a sharp, irritating odor. Its melting point is around 8.4°C, and its boiling point is about 100.8°C. Formic acid is highly miscible with water, alcohols, and other polar solvents, due to its small size and hydrogen bonding capabilities. As a strong organic acid, it ionizes readily in aqueous solutions, giving it a low pH. Chemically, formic acid can undergo reactions like esterification, reduction, and formation of formate salts, demonstrating its versatility in organic synthesis and industrial processes.
How Formic Acid (HCOOH) is Synthesized
In modern industry, formic acid production primarily involves the hydrolysis of methyl formate. Which is derived from methanol and carbon monoxide. This efficient process ensures high yields and cost-effectiveness. Historically, formic acid obtained by distilling ants or fermenting organic matter,these methods are rarely used today. HCOOH can be synthesized by oxidizing formaldehyde or through formylation reactions. Sustainability efforts have driven research into greener synthesis routes using biomass feedstocks or carbon dioxide conversion, which could make formic acid production more eco-friendly in the future.
Uses and Applications of Formic Acid
Formic acid (HCOOH) plays a critical role in various industries due to its versatility. In the textile industry, it serves as a dye-fixing agent. In leather processing, formic acid aids in tanning and pH regulation. Agriculture uses formic acid to preserve silage, helping maintain feed quality for livestock. Additionally, formic acid’s antibacterial properties make it valuable in animal feed formulations. In the pharmaceutical industry, formic acid serves as a precursor for various compounds. Its use in fuel cells, where HCOOH acts as a hydrogen carrier, highlights its potential in sustainable energy technologies.
Formic Acid in Nature: Ants, Bees, and Beyond
Formic acid is best known as a defense mechanism in ants and certain species of bees. Ant venom contains concentrated HCOOH, which deters predators and aids in territorial disputes. Beyond insects, small amounts of formic acid appear in some plants, especially during metabolic processes involving carbon fixation. Certain microbes also produce formic acid during fermentation. The natural occurrence of formic acid illustrates its importance in ecological balance, where it mediates species interactions and microbial metabolism, demonstrating the versatility of this simple yet powerful organic acid.
Health and Safety Concerns Related to Formic Acid
Despite its usefulness, formic acid (HCOOH) poses some health risks, particularly in concentrated forms. Inhalation can irritate the respiratory tract, while skin contact may cause burns or dermatitis. Prolonged exposure can damage eyes and mucous membranes. Safe handling practices include using gloves, goggles, and adequate ventilation when working with formic acid. Regulatory guidelines outline exposure limits in occupational settings, ensuring worker safety. Understanding these safety measures helps balance the benefits and risks associated with formic acid use.
Environmental Impact of Formic Acid
Formic acid is considered biodegradable and environmentally friendly in small amounts. Microorganisms readily break down HCOOH into carbon dioxide and water, reducing its persistence in the environment. However, industrial spills or overuse can lower pH in local ecosystems, potentially harming aquatic life. Regulatory agencies monitor industrial emissions of formic acid to minimize environmental risks. Recent studies highlight the role of formic acid in atmospheric chemistry, where it contributes to organic aerosol formation. With careful management, formic acid can be used safely with minimal long-term environmental impact.
Common Myths and Misconceptions About Formic Acid
There are several misconceptions surrounding formic acid (HCOOH). One common myth is that formic acid is the same as formaldehyde — they are related but distinct compounds. Another misconception is that formic acid is only found in ants, though it appears in various natural and industrial processes. Some believe formic acid is highly toxic, but low concentrations are relatively safe. Understanding these myths helps clarify HCOOH’s real-world applications and risks. Accurate information about formic acid promotes safer handling and better appreciation of its value across industries.
Comparing Formic Acid with Other Carboxylic Acids
Compared to other carboxylic acids, formic acid (HCOOH) stands out due to its small molecular size and strong acidity. Formic acid is more acidic than acetic acid because its formyl group enhances proton donation. While acetic acid is widely used in food preservation, formic acid’s applications lean toward industrial and agricultural sectors. Both acids participate in esterification and oxidation reactions, but formic acid’s smaller size makes it more reactive. Understanding these differences helps chemists and manufacturers choose the right acid for specific applications, whether in organic synthesis, food production, or industrial processing.
See Also: How Many Ribs Does a Person Have
FAQ’s
Q1. Is formic acid organic or inorganic?
Formic acid is an organic compound, classified as the simplest carboxylic acid.
Q2. What is the pH of formic acid?
The pH varies depending on concentration, but a 0.1M solution has a pH around 2.4.
Q3. Is formic acid flammable?
Yes, concentrated formic acid is flammable and should be kept away from ignition sources.
Q4. How does formic acid affect skin?
Concentrated HCOOH can cause burns and skin irritation; protective gear is recommended.
Conclusion
Formic acid (HCOOH) is a fascinating compound with significant roles in nature, industry, and research. From its origins in ant venom to its applications in textiles, agriculture, and fuel cells, formic acid demonstrates exceptional versatility. Its unique combination of acidity, reactivity, and natural occurrence makes it indispensable in numerous fields. While it poses some safety risks, careful handling ensures its benefits far outweigh potential hazards. As researchers explore greener synthesis methods and new applications, formic acid’s importance is set to grow, securing its place as one of the most valuable small organic acids in chemistry and industry